For those of you who dont know this there are more reasons for using sorbate then just to make sure your wine doesnt start fermenting again in the bottle after sweetening a wine. There are reasons to use it even if you havent sweeten your wine. Here is an article copied from Wikipedia for you to help understand this a little better. I know Masta posted something like this quite awhile ago but sometimes stuff gets forgotton and there are many new winemakers who could benefit from this.
<h1 id="firsting" ="firsting">Potassium sorbate</h1>
<h3 id="siteSub">From Wikipedia, the free encyclopedia</h3>
<div id="jump-to-nav">Jump to: navigation, search
<table ="toccolours" style="margin: 0pt 0pt 1em 1em; : right; clear: right; border-collapse: collapse;" width="250" border="1">
<t><tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; width: 30%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Potassium sorbate<sup id="cite_ref-0" ="reference">[</span>1]</span></sup><sup id="cite_ref-1" ="reference">[</span>2]</span></sup></th>
</tr>
<tr>
<td colspan="2" align="center" ="#ffffff">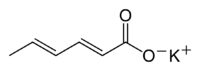 </td>
</td>
</tr>
<tr>
<td>IUPAC name</td>
<td>
<div id="Nav1" ="Nav" style="border: medium none ; padding: 0pt;">
<div ="Nav" style=": transparent none repeat scroll 0% 0%; width: 100%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial;" align="left">[show]
<div ="NavContent" style="text-align: left; display: none;">
<div style="overflow: auto; width: 100%;">Potassium (2E,4E)-hexa-2,4-dienoate
</td>
</tr>
<tr>
<td>Other names</td>
<td>E202
Sorbistat-K
Sorbistat potassium</td>
</tr>
<tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Identifiers</th>
</tr>
<tr>
<td ="">CAS number</td>
<td ="">24634-61-5</td>
</tr>
<tr>
<td>PubChem</td>
<td>23676745</span></td>
</tr>
<tr>
<td>SMILES</td>
<td>
<div id="Nav2" ="Nav" style="border: medium none ; padding: 0pt;">
<div ="Nav" style=": transparent none repeat scroll 0% 0%; width: 100%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial;" align="left"><tt></tt>[show]
<div ="NavContent" style="text-align: left; display: none;">
<div style="overflow: auto; width: 100%;"><tt>C\C=C\C=C\C(=O)[O-].[K+]</tt>
</td>
</tr>
<tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Properties</th>
</tr>
<tr>
<td>Molecular formula</td>
<td>C<sub>6</sub>H<sub>7</sub>KO<sub>2</sub></td>
</tr>
<tr>
<td>Molar mass</td>
<td>150.22 g/mol</td>
</tr>
<tr>
<td>Density</td>
<td>1.363 g/cm<sup>3</sup></td>
</tr>
<tr>
<td>Melting point</td>
<td>
270 °C (decomposition)
</td>
</tr>
<tr>
<td>Solubility in water</td>
<td>58.2% at 20 °C</td>
</tr>
<tr>
<td style=": rgb(248, 234, 186) none repeat scroll 0% 0%; width: 30%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Except where noted otherwise, data are given for
materials in their <a href="http://en.wikipedia.org/wiki/Standard_state" target="_blank">standard state
(at 25°C, 100kPa)</a>
Infobox references</td>
</tr>
</t></table>
Potassium sorbate is the potassium salt of sorbic acid. Its primary use is as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal care.
<table id="toc" ="toc" summary="Contents">
<t><tr>
<td>
<div id="toctitle">
<h2>Contents</h2>
[hide]</span>
<ul><li ="toclevel-1">1</span> Chemistry</span><li ="toclevel-1">2</span> Use</span><li ="toclevel-1">3</span> Toxicology</span><li ="toclevel-1">4</span> See also</span><li ="toclevel-1">5</span> References</span>[/list]
</td>
</tr>
</t></table>
//<![C[
if (.showTocToggle) { var tocShowText = "show"; var tocHideText = "hide"; showTocToggle(); }
//]]>
<a name="Chemistry" id="Chemistry"></a>
<h2>[edit]</span> Chemistry</span></h2>
The molecular formula of potassium sorbate is C<sub>6</sub>H<sub>7</sub>O<sub>2</sub>K and its systematic name is potassium (E,E)-hexa-2,4-dienoate. Its has a molecular weight of 150.22 g/mol. It is very soluble in water (58.2% at 20 °C). It is prepared by the reaction of sorbic acid with potassium hydroxide.
<a name="Use" id="Use"></a>
<h2>[edit]</span> Use</span></h2>
Potassium sorbate is used to inhibit molds and yeasts in many foods, such as cheese, wine, yogurt, dried meats, apple cider and baked goods. It can also be found in the ingredients list of many dried fruit
products. In addition, herbal dietary supplement products generally
contain potassium sorbate, which acts to prevent mold and microbes and
to increase shelf life, and is used in quantities at which there are no
known adverse health effects.<sup ="noprint Template-Fact">[citation needed]</span></sup> Labeling of this preservative reads as "potassium sorbate" on the ingredient statement. Also, it is used in many personal care products to inhibit the development of microorganisms for shelf stability. Some manufacturers are using this preservative as a replacement for parabens.
Also known affectionately as "wine stabilizer", potassium sorbate produces sorbic acid when added to wine. It serves two purposes. When active fermentation
has ceased and the wine is racked for the final time after clearing,
potassium sorbate will render any surviving yeast incapable of
multiplying. Yeast living at that moment can continue fermenting any
residual sugar into CO<sub>2</sub> and alcohol,
but when they die no new yeast will be present to cause future
fermentation. When a wine is sweetened before bottling, potassium
sorbate is used to prevent refermentation when used in conjunction with
potassium metabisulfite. It is primarily used with sweet wines, sparkling wines, and some hard ciders but may be added to table wines which exhibit difficulty in maintaining clarity after fining.
Some molds (notably some Trichoderma and Penicillium strains) and yeasts are able to detoxify sorbates by decarboxylation, producing 1,3-pentadiene. The pentadiene manifests as a typical odor of kerosene or petroleum.<sup id="cite_ref-2" ="reference">[</span>3]</span></sup>
<a name="Toxicology" id="Toxicology"></a>
<h2>[edit]</span> Toxicology</span></h2>
Potassium sorbate is considered to be safe because of its long term
safety record and non-toxic profile. Potassium sorbate is
non-irritating and non-sensitizing. Allergic reactions are rare<sup ="noprint Template-Fact">[citation needed]</span></sup> and it is well tolerated when administered internally.<sup id="cite_ref-3" ="reference">[</span>4]</span></sup>
<h1 id="firsting" ="firsting">Potassium sorbate</h1>
<h3 id="siteSub">From Wikipedia, the free encyclopedia</h3>
<div id="jump-to-nav">Jump to: navigation, search
<table ="toccolours" style="margin: 0pt 0pt 1em 1em; : right; clear: right; border-collapse: collapse;" width="250" border="1">
<t><tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; width: 30%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Potassium sorbate<sup id="cite_ref-0" ="reference">[</span>1]</span></sup><sup id="cite_ref-1" ="reference">[</span>2]</span></sup></th>
</tr>
<tr>
<td colspan="2" align="center" ="#ffffff">
 </td>
</td></tr>
<tr>
<td>IUPAC name</td>
<td>
<div id="Nav1" ="Nav" style="border: medium none ; padding: 0pt;">
<div ="Nav" style=": transparent none repeat scroll 0% 0%; width: 100%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial;" align="left">[show]
<div ="NavContent" style="text-align: left; display: none;">
<div style="overflow: auto; width: 100%;">Potassium (2E,4E)-hexa-2,4-dienoate
</td>
</tr>
<tr>
<td>Other names</td>
<td>E202
Sorbistat-K
Sorbistat potassium</td>
</tr>
<tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Identifiers</th>
</tr>
<tr>
<td ="">CAS number</td>
<td ="">24634-61-5</td>
</tr>
<tr>
<td>PubChem</td>
<td>23676745</span></td>
</tr>
<tr>
<td>SMILES</td>
<td>
<div id="Nav2" ="Nav" style="border: medium none ; padding: 0pt;">
<div ="Nav" style=": transparent none repeat scroll 0% 0%; width: 100%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial;" align="left"><tt></tt>[show]
<div ="NavContent" style="text-align: left; display: none;">
<div style="overflow: auto; width: 100%;"><tt>C\C=C\C=C\C(=O)[O-].[K+]</tt>
</td>
</tr>
<tr>
<th style=": rgb(248, 234, 186) none repeat scroll 0% 0%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Properties</th>
</tr>
<tr>
<td>Molecular formula</td>
<td>C<sub>6</sub>H<sub>7</sub>KO<sub>2</sub></td>
</tr>
<tr>
<td>Molar mass</td>
<td>150.22 g/mol</td>
</tr>
<tr>
<td>Density</td>
<td>1.363 g/cm<sup>3</sup></td>
</tr>
<tr>
<td>Melting point</td>
<td>
270 °C (decomposition)
</td>
</tr>
<tr>
<td>Solubility in water</td>
<td>58.2% at 20 °C</td>
</tr>
<tr>
<td style=": rgb(248, 234, 186) none repeat scroll 0% 0%; width: 30%; -moz--clip: -moz-initial; -moz--origin: -moz-initial; -moz--inline-policy: -moz-initial; text-align: center;" colspan="2">Except where noted otherwise, data are given for
materials in their <a href="http://en.wikipedia.org/wiki/Standard_state" target="_blank">standard state
(at 25°C, 100kPa)</a>
Infobox references</td>
</tr>
</t></table>
Potassium sorbate is the potassium salt of sorbic acid. Its primary use is as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal care.
<table id="toc" ="toc" summary="Contents">
<t><tr>
<td>
<div id="toctitle">
<h2>Contents</h2>
[hide]</span>
<ul><li ="toclevel-1">1</span> Chemistry</span><li ="toclevel-1">2</span> Use</span><li ="toclevel-1">3</span> Toxicology</span><li ="toclevel-1">4</span> See also</span><li ="toclevel-1">5</span> References</span>[/list]
</td>
</tr>
</t></table>
//<![C[
if (.showTocToggle) { var tocShowText = "show"; var tocHideText = "hide"; showTocToggle(); }
//]]>
<a name="Chemistry" id="Chemistry"></a>
<h2>[edit]</span> Chemistry</span></h2>
The molecular formula of potassium sorbate is C<sub>6</sub>H<sub>7</sub>O<sub>2</sub>K and its systematic name is potassium (E,E)-hexa-2,4-dienoate. Its has a molecular weight of 150.22 g/mol. It is very soluble in water (58.2% at 20 °C). It is prepared by the reaction of sorbic acid with potassium hydroxide.
<a name="Use" id="Use"></a>
<h2>[edit]</span> Use</span></h2>
Potassium sorbate is used to inhibit molds and yeasts in many foods, such as cheese, wine, yogurt, dried meats, apple cider and baked goods. It can also be found in the ingredients list of many dried fruit
products. In addition, herbal dietary supplement products generally
contain potassium sorbate, which acts to prevent mold and microbes and
to increase shelf life, and is used in quantities at which there are no
known adverse health effects.<sup ="noprint Template-Fact">[citation needed]</span></sup> Labeling of this preservative reads as "potassium sorbate" on the ingredient statement. Also, it is used in many personal care products to inhibit the development of microorganisms for shelf stability. Some manufacturers are using this preservative as a replacement for parabens.
Also known affectionately as "wine stabilizer", potassium sorbate produces sorbic acid when added to wine. It serves two purposes. When active fermentation
has ceased and the wine is racked for the final time after clearing,
potassium sorbate will render any surviving yeast incapable of
multiplying. Yeast living at that moment can continue fermenting any
residual sugar into CO<sub>2</sub> and alcohol,
but when they die no new yeast will be present to cause future
fermentation. When a wine is sweetened before bottling, potassium
sorbate is used to prevent refermentation when used in conjunction with
potassium metabisulfite. It is primarily used with sweet wines, sparkling wines, and some hard ciders but may be added to table wines which exhibit difficulty in maintaining clarity after fining.
Some molds (notably some Trichoderma and Penicillium strains) and yeasts are able to detoxify sorbates by decarboxylation, producing 1,3-pentadiene. The pentadiene manifests as a typical odor of kerosene or petroleum.<sup id="cite_ref-2" ="reference">[</span>3]</span></sup>
<a name="Toxicology" id="Toxicology"></a>
<h2>[edit]</span> Toxicology</span></h2>
Potassium sorbate is considered to be safe because of its long term
safety record and non-toxic profile. Potassium sorbate is
non-irritating and non-sensitizing. Allergic reactions are rare<sup ="noprint Template-Fact">[citation needed]</span></sup> and it is well tolerated when administered internally.<sup id="cite_ref-3" ="reference">[</span>4]</span></sup>




















































